Fda Label Expansion . in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its. A transition period for labeling will last three years after the effective date. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the.
from www.drugwatch.com
announced just more than a year after astrazeneca announced topline results, the fda has approved a label. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. A transition period for labeling will last three years after the effective date. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older.
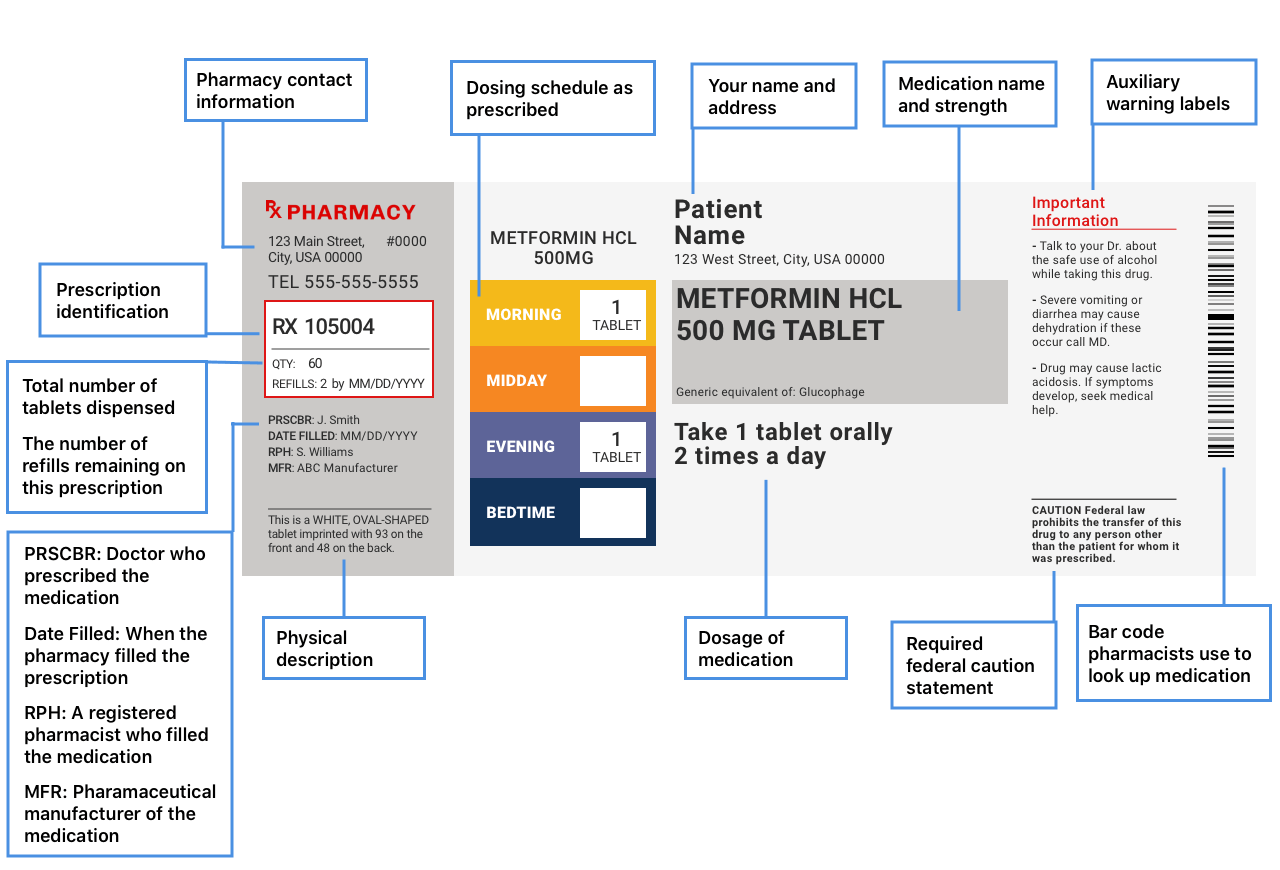
How to Read OvertheCounter and Prescription Drug Labels
Fda Label Expansion the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. A transition period for labeling will last three years after the effective date. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its.
From www.msn.com
Quidel gets FDA okay for Savanna platform, HSV 1+2/VZV assay Fda Label Expansion announced just more than a year after astrazeneca announced topline results, the fda has approved a label. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its.. Fda Label Expansion.
From www.greatplacetowork.com
Working at Esperion Great Place To Work® Fda Label Expansion the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. A transition period for labeling will last three years after the effective date. in a development that's set to. Fda Label Expansion.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Label Expansion A transition period for labeling will last three years after the effective date. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. announced just. Fda Label Expansion.
From www.pharmaceutical-technology.com
Novartis bags paediatric FDA label expansion for Lutathera Fda Label Expansion typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4. Fda Label Expansion.
From www.pharmaceutical-technology.com
FDA approves label expansion for Novartis’ Leqvio Pharmaceutical Fda Label Expansion announced just more than a year after astrazeneca announced topline results, the fda has approved a label. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have. Fda Label Expansion.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Label Expansion A transition period for labeling will last three years after the effective date. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. in a development that's set to. Fda Label Expansion.
From lifesciences.csoftintl.com
Merck Granted Label Expansion by FDA for Keytruda Fda Label Expansion A transition period for labeling will last three years after the effective date. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. announced just more than. Fda Label Expansion.
From www.tctmd.com
FDA Perspectives on TAVR Label Expansion Using RealWorld Evidence Fda Label Expansion A transition period for labeling will last three years after the effective date. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. in a development that's set to. Fda Label Expansion.
From www.cardiometabolichealth.org
FDA Expands Labels for Bempedoic Acid and Ezetimibe Tablets Fda Label Expansion typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. A transition period for labeling will last three years after the effective date. the fda has expanded the label. Fda Label Expansion.
From www.pharmaceutical-technology.com
Roche wins second FDA label expansion for Alecensa in NSCLC Fda Label Expansion A transition period for labeling will last three years after the effective date. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its. the fda has expanded. Fda Label Expansion.
From www.pharmashots.com
Sarepta Therapeutics’ Elevidys Gains the US FDA’s Label Expansion Fda Label Expansion typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients. Fda Label Expansion.
From seekingalpha.com
Enhertu under FDA priority review for label expansion (NASDAQAZN Fda Label Expansion announced just more than a year after astrazeneca announced topline results, the fda has approved a label. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. A transition period for labeling will last three years after the effective date. the fda has expanded the label. Fda Label Expansion.
From www.msn.com
Bristol Myers wins FDA review for label expansion of lung cancer therapy Fda Label Expansion typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. A transition period for labeling will last three years after the effective date. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. in a. Fda Label Expansion.
From newsfilter.io
BioMarin, Pioneer in Rare Disease Treatments for Phenylketonuria (PKU Fda Label Expansion A transition period for labeling will last three years after the effective date. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its. announced just more than. Fda Label Expansion.
From telixpharma.com
FDA Approves Expanded Indication for Telix’s Illuccix® to Include Fda Label Expansion in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical. Fda Label Expansion.
From meyers.com
13 Most Important Parts of a Product Label Meyers Fda Label Expansion the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. A transition period for labeling will last three years after the effective date. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. typically, when fda approves an expansion. Fda Label Expansion.
From www.pharmaceutical-technology.com
Amarin Vascepa FDA to convene meeting label expansion Fda Label Expansion typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. announced just more than a year after astrazeneca announced topline results, the fda has approved a label. in a development that's set to change the lives of many patients, blueprint medicines’ ayvakit has finally won its.. Fda Label Expansion.
From www.slideteam.net
Top 10 Drug Development Timeline PowerPoint Presentation Templates in 2024 Fda Label Expansion A transition period for labeling will last three years after the effective date. typically, when fda approves an expansion of the indication for a drug, it means that additional clinical data have shown the. the fda has expanded the label for sarepta's duchenne muscular dystrophy gene therapy elevidys to all patients age 4 and older. in a. Fda Label Expansion.